JN and CP equally contributed
Introduction
In the setting of clinical trials Venetoclax (VEN) combined with hypomethylating agents (HMAs) has shown fair activity in R/R AML and impressive results in treatment-naïve elderly AML patients. However, no clear guidelines are available on real-life management, especially in the outpatient setting. This study involving R/R AML patients treated with VEN combined with HMAs aims to amelioratate physicians' knowledge about the administration of these regimens.
Methods
This is a single-center retrospective study involving AML patients treated with Venetoclax combined with HMAs. Data were collected in accordance with GCP and Helsinky declaration. Adverse events (AEs) were graded according to CTCAE v4.03. Survival is estimated with Kaplan-Meyer method.
Results
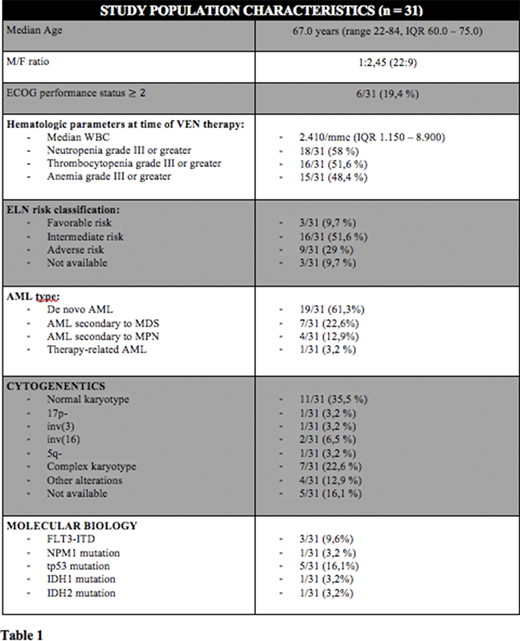
Thirty-one R/R AML patients have been treated with VEN plus HMAs from March 2018 to March 2020 and completed at least 1 therapy course (range 1-9, median 2, IQR 1.0 - 5.0) (Table 1: patients' characteristics).
Seventeen out of 31 (54,8 %) had received intensive chemotherapy as induction therapy. Twenty-two patients (71 %) had already received HMAs therapy, of which 14/31 (45,2 %) as first and only previous line of therapy. VEN was combined with azacitidine in 13/31 (41,9%) and with decitabine in 18/31 patients (58.1 %). The majority of patients (22/31, 70,9%) received the first cycle as out-patients, special focus of our analysis. For clinical reasons, only 9 out of 31 (29,1 %) patients were hospitalized and were used as control.
In the outpatients setting, VEN dose escalation was managed with at least a weekly laboratory and clinical monitoring and the drug was often increased more slowly to carefully prevent tumor lysis syndrome or other complications. Specifically, there has been a trend toward a ramp-up schedule different from that indicated in clinical trials in the outpatient setting in comparison with hospitalized patients (77,2 % vs 33,3 %).
In term of safety, no cases of tumor lysis syndrome (TLS) and only 2 AEs were documented during ramp-up phase, both experienced by hospitalized patients. Seventeen AEs were documented during cycle 1, affecting more frequently hospitalized patients (77,9% vs 31,8 %). In the outpatient setting, the early 30-days and 60-days mortalities were 4,5 % (1/22) and 13,6 % (3/22), respectively, comparable to percentages documented in hospitalized subgroup (0% and 11,1%).
Overall, with a median follow-up of 138 days (IQR 69 - 285), we reported 48 AEs, of which 28 were grade III-IV and the most common were hematological (13/28, 48,1 %) or infective (14/28, 51,8 %). Twenty out of 31 (64,5 %) patients reduced VEN dosage during treatment, of which 12/20 (60 %) due to occurring AEs, and remaining patients for azole coadministration. Eleven out of 31 (35,5 %) patients required hospitalization, specifically 3 out of 9 hospitalized patients (33,3 %) during the subsequent outpatient phase of treatment, and 8/22 (36,3 %) patients who underwent VEN therapy outpatient, of which only 2 during the first 28 days of treatment. Twenty-four and 5 AEs were followed by a VEN temporary (median duration 14 days, range 5-120) and permanent withdrawn, respectively.
As for the rate of response, the Overall Response Rate (ORR) by VEN plus HMAs therapy in our R/R study population, defined as CR + CRi + HI, was 41,9 % (13/31), with a CR/CRi rate of 22,6 % (7/31). The median time to first response was 67.0 days (IQR 37.0 - 133.5) and the median duration of response was 131.0 days (IQR 89.0 - 151.0). Four out of 31 (12,9 %) patients received subsequent HSCT. The median OS was 285 days (95% C.I. 178 - 392), with no difference in OS between patients who underwent VEN plus HMAs outpatient and patients who underwent the first cycle hospitalized (p = 0,38).
Conclusions
With the limitations of a single-center retrospective study, our real-life data indicate that VEN plus HMAs is feasible in an outpatient management, without TLS or other limiting toxicities and with comparable early-mortality and toxicity profiles, even in the ramp-up phase and first therapeutic cycle. There was no significant impact of the outpatient management on treatment effectiveness, with data in line with published R/R AML cohorts. Further studies evaluating the clinical, social and economic impact of outpatient VEN-based treatments are highly warranted.
Papayannidis:Abbvie, Janssen, Novartis, Amgen, Pfizer:Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.Cavo:Jannsen, BMS, Celgene, Sanofi, GlaxoSmithKline, Takeda, Amgen, Oncopeptides, AbbVie, Karyopharm, Adaptive:Consultancy, Honoraria.
In R/R AML, available data regarding the combination of VEN plus HMAs come only from retrospective studies where VEN is administered as an off-label prescription due to its widespread approval for chronic lymphocytic leukemia due to promising results obtained in treatment-naive elderly AML patients
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal